Which Set of Quantum Numbers Is Not Possible
Can be 1 to 7 l Secondary Quantum NumberOrbital Shape Quantum number. By solving the Schrödinger equation Hy Ey we obtain a set of mathematical equations called wave functions y which describe the probability of finding electrons at certain energy levels within an.

How To Determine The Maximum Number Of Electrons Given A Set Of Quantum Numbers Youtube
We exclude molecules where the relaxation did not converge or where the bonding pattern changed during relaxation.

. A quantized system requires at least one. No it is not possible. A quantum of energy is the least amount possible or the least extra amount and quantum mechanics describes how that.
Table of Allowed Quantum Numbers Writing Electron Configurations Properties of Monatomic Ions References Quantum Numbers and Atomic Orbitals. Yes it is possible to have two electrons with the same n l and m_l. A n4 l0 n 4 l 0 This represents the 4s orbital.
Quantum mechanics provide information on the probability of measuring a given value which can be interpreted as follows. The tally of quantum numbers varies from system to system and has no universal answer. The value should be be either -1 0 or 1.
They are relaxed using the B3LYP functional with the 6-31G2dp basis set as implemented in the ORCA quantum chemistry package. Quantum numbers describe specific properties of an electron. QUANTUM NUMBERS WORKSHEET 1.
The spin of one electron must be 12 while the spin of the other electron must be -12. It is thus difficult to list all possible quantum numbers. Only one electron in a given atom can have the set of quantum numbers given in the question.
State the four quantum numbers then explain the possible values they may have and what they actually represent. Since photons are discontinuous it is not possible to use a classical deterministic theory but only a probabilistic and statistical theory. Learn about atomic orbital the four quantum numbers principal angular momentum magnetic and spin and how to write quantum.
There are three subshells in principal. N 0 l 0 ml 0 ms 1 View Answer Two. All s orbitals can only hold up to 2 electrons regardless of the principal quantum number.
We report the electronic energy the dipole moment the energies of the HOMO and LUMO orbitals as well as the HOMO-LUMO gap. Quantum mechanics explain how the universe works at a scale smaller than atoms. Represents the shape of the orbital- s p f d.
State whether or not the following set of quantum numbers would be possible for an electron in an atom. More than fifty years since the introduction of this model there is an increase in the number of works that use kicked rotor model as a fundamental template to study a variety of questions in nonlinear dynamics quantum chaos condensed matter physics and. It is also called quantum physics or quantum theoryMechanics is the part of physics that explains how things move and quantum is the Latin word for how much.
Quantum numbers needed for a given system. Having infinite identical systems available and performing the same measurement on all systems the. Any quantum system can have one or more quantum numbers.
How many electrons in an atom may have the quantum numbers n 4 and 1 0. Quantum logic gates are represented by unitary matricesA gate which acts on qubits is represented by a unitary matrix and the set of all such gates with the group operation of matrix multiplication is the symmetry group U2 nThe quantum states that the gates act upon are unit vectors in complex dimensions with the complex Euclidean norm the 2-norm. Although complex numbers are essential in mathematics they are not needed to describe physical experiments as those are expressed in terms of probabilities hence real numbers.
Hence these parameters must be found for each system to be analyzed. Each electron in an atom has a unique set of quantum numbers. N Pricipal Quantum Number.
Kicked rotor is a paradigmatic model for classical and quantum chaos in time-dependent Hamiltonian systems. Represents the energy level the electron is in linked to the periods of the periodic. M_l3 is not in the range of -l to l.

The Correct Set Of Quantum Numbers For Valence Electron Of Rb Atomic Number 37 Is

Ex 2 1 2 If Set A Has 3 Elements And B 3 4 5 Find Number

The Pauli Exclusion Principle Physics
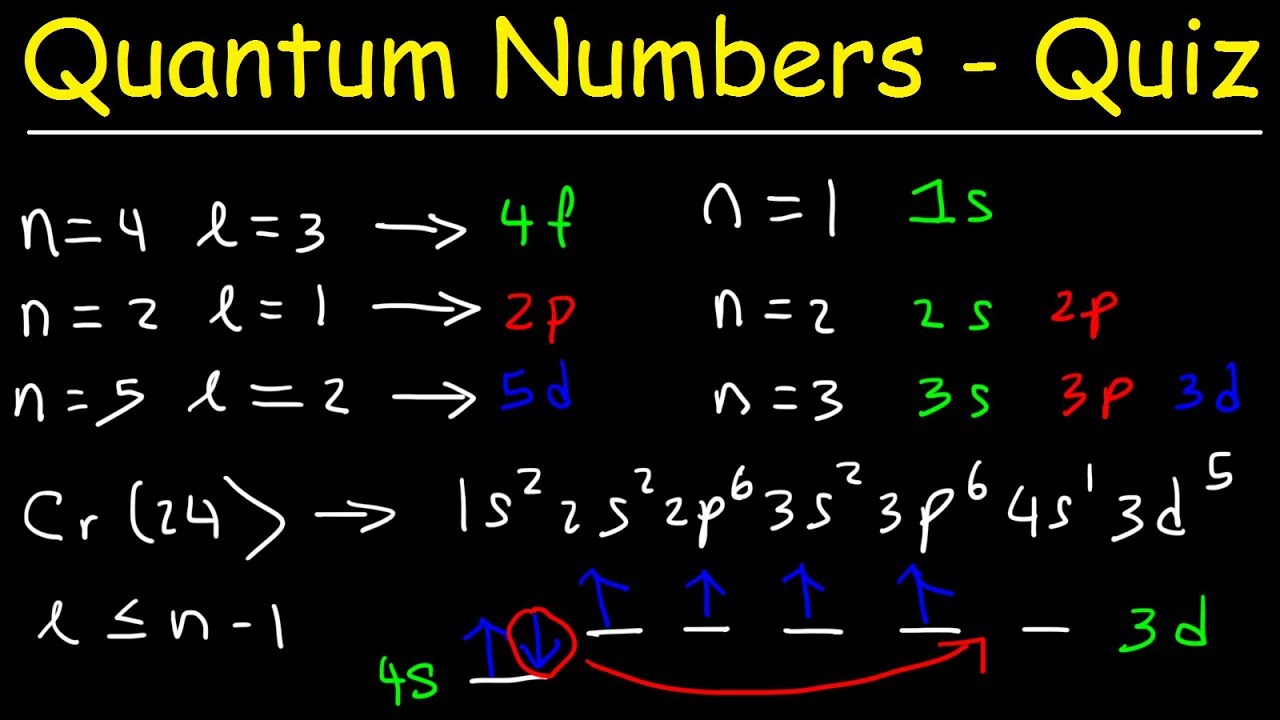
Quantum Numbers N L Ml Ms Spdf Orbitals Youtube

Number Sets In Use ℍ Quaternions Have A Different Story ℍ Are Not Required To Solve Any Algebraic Equations Hyper Algebra Equations Equations Solving

Number Sets Characteristics Examples Video Lesson Transcript Study Com

How To Determine The Maximum Number Of Electrons Given A Set Of Quantum Numbers Youtube

Amorite Israelite Slingers In 2022 History 20th Century Mystery Of History Ancient

Seven Crystal System Chemistry Study Guide Teaching Chemistry Chemistry Classroom

Permutations R Permutation Aka Ordered R Selection An Ordered Arrangement Of R Elements Of A Set Of N Dis Discrete Mathematics College Subjects Mathematics

Quantum Numbers The Easy Way Youtube
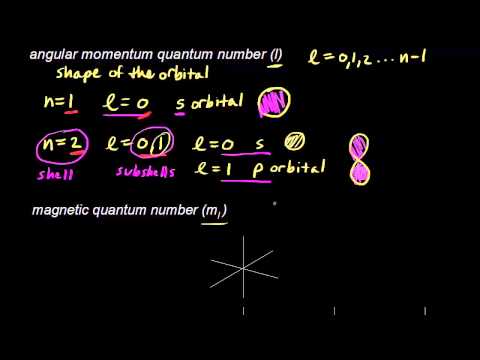
Quantum Numbers Video Quantum Physics Khan Academy

How To Determine The Maximum Number Of Electrons Using Allowed Quantum Numbers 8 Cases Youtube

How To Determine The Maximum Number Of Electrons Using Allowed Quantum Numbers 8 Cases Youtube
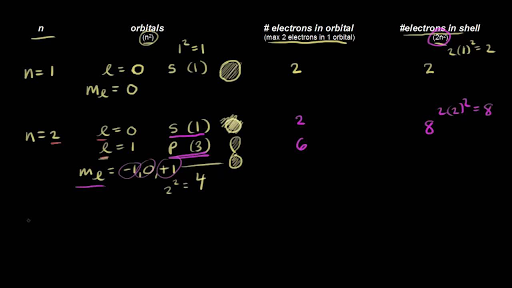
Quantum Numbers For The First Four Shells Video Khan Academy

Perfect Squares And Sums Of Odd Numbers Odd Numbers Perfect Squares Numbers


Comments
Post a Comment